In this day and age, it is almost impossible to find a production process in the food, cosmetic or pharmaceutical industries that does not include extraction processes.
Discovery of supercritical state using a gun barrel
The supercritical state of fluids was discovered by the French Baron Charles Cagniard de la Tour in 1822, although the true significance of his discovery and the possibilities it opens up became apparent only later. Cagniard, an engineer and physicist,was curious and wanted to find out what happens to a liquid if boiled in a closed vessel. For the purposes of this experiment, he put some liquid and a ball of flint into a small closed pressure vessel (Papin's digester), which he created using the end of a very thick gun barrel. He then listened to the noise the ball generated while rolling in the cold barrel and when the barrel was heated. He noticed that above a certain pressure and temperature, the splashing sound the ball made as it moved in and out of the liquid disappeared. This led him to believe that the liquid and gas densities in the vessel became equal and that a single state was effectively formed ... This was the first step towards discovering a phenomenon we now call the supercritical state. This term refers to the state a substance reaches above its critical point of temperature and pressure, in which the liquid-gas phase boundary becomes blurred.

Gas liquefaction and designing the Titanic?
In 1869, chemist and physicist Thomas Andrews continued the research on the density of carbon dioxide. He was the first to work with binary mixtures of carbon dioxide and nitrogen. His reputation mainly rests on his work with gas liquefaction. He conducted extensive research on gas laws and studied the relationship between the pressure, temperature, and volume of carbon dioxide. Andrews established the concept of critical temperature and critical pressure, which shows that a substance can continuously pass from the gaseous to the liquid state. He also proved that carbon dioxide can be carried from any liquid state to a gaseous state with no loss of homogeneity. However, despite all his achievements, at hearing the name Thomas Andrews many first think of the naval architect who designed the great ocean liner Titanic and died when it sank in 1912 during its maiden voyage.

145 years of liquid oxygen
In the following years, numerous scientists attempted and managed to liquefy various other gases. In 1877, Louis Paul Cailletet was the first to liquefy oxygen. James Ballantyne Hannay was among the first to prove the solubility of solids in supercritical gases.
In the late 1970s, new extraction and separation technologies advanced significantly. The first breakthrough in the research of supercritical CO2 extraction systems was achieved in Germany on an experimental high-pressure device.
Carbon dioxide for improved oil recovery
CO2 capture technology has been used since the 1920s to separate the carbon dioxide sometimes found in natural gas reservoirs from the saleable methane gas. In the US, carbon dioxide has been captured and injected into oil fields since the early 1970s to enhance oil recovery.

Source: cen.acs.org
Decaffeinated coffee and non-bitter beer
The German scientist Kurt Zosel is credited with the first U.S. patent for the decaffeination of coffee (1970) using supercritical fluid extraction. According to the patent specification, green coffee beans must first be steamed, then the supercritical CO2 is pumped through them. The caffeine dissolves in the supercritical CO2 and is then drawn out of the beans. This process can selectively reduce the amount of caffeine from the initial 3 % to less than 0.02 % without removing any of the substances that contribute to the aroma produced by roasting. The removed caffeine can be isolated for further use (e.g. in the pharmaceutical industry or in the production of beverages) using additional water which is pumped through an activated carbon filter or alternatively by carrying out the process of distillation, crystallization, or reverse osmosis.

After a series of studies, CO2 extraction systems became widely used in various fields.
Nowadays, supercritical fluids are used for a variety of purposes: for the extraction of flavors and fragrances from plants, in the food industry for the removal of fats and oils of plant and animal origin (e.g. cocoa butter and soybean oil), for the extraction of the aromatic components of hops, the removal of the bitter extract from beer and caffeine from tea, and for the extraction of essential oils from aromatic herbs.
A wide range of other uses of this modern technology were studied in the field of chemical reactions, separation, and purification; it has also made great strides in medicine, pharmacy, the chemical industry and food and environment protection.
|
Supercritical fluids have low viscosity, density, and surface tension, which means that they can effuse through solids, like a gas. When supercritical fluids pass through the pores of a solid substance, they can selectively dissolve components contained within the solid substance, much like a liquid. The dissolved components are then removed from the solid and can be isolated by relieving the supercritical fluid and thus returning it to the gaseous state and "depositing" the extracted substance. This results in little or no solvent residue in the extracted product. The gas can then be returned to the supercritical state and reused. An additional advantage of this method is the short extraction times, as the extraction process is significantly faster due to the increased diffusivity and lower viscosity of supercritical CO2 compared to other liquids. Supercritical fluids can also have very different properties compared to their normal state: water in the supercritical state differs from ordinary water in that it is nonpolar and acidic. |
The most sought-after fluid
By the late 1970s, carbon dioxide had become the most sought-after fluid due to its low critical temperature (31.1 °C) and relatively low critical pressure (7.38 MPa). In addition, it is a nontoxic, nonflammable, noncorrosive, inexpensive and widely available gas that is easy to handle. As a result, it is recognized as a green solvent. If properly purified, it has a minimal effect on the quality of the extracted biocomponents, thus preserving their medicinal and functional properties.
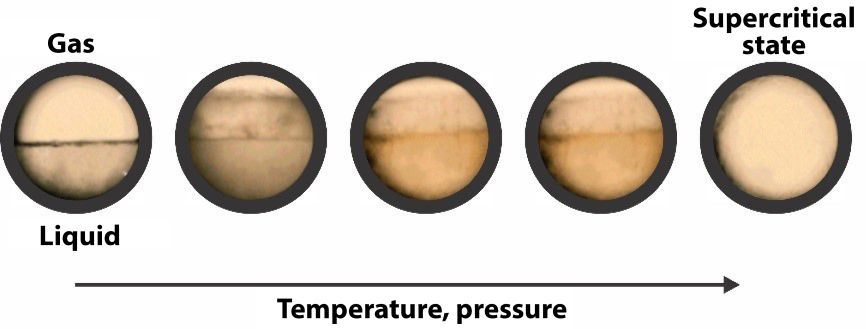
A frozen gas
In 1835, Humphry Davy and Michael Faraday were the first to liquefy CO2. Carbon dioxide is a natural compound that can be found all around us. It is indispensable for plants, as they need it in order to carry out the photosynthesis process. Although normally in the gaseous state, it can also be frozen and compressed into a liquid. Today, CO2 has found its place in the chemical, oil, and food industries.
Under normal temperature and pressure conditions, carbon dioxide usually behaves like a gas. If frozen, it appears in a solid state and is also called dry ice. If the temperature and pressure are above their standard values or above the critical point, carbon dioxide behaves both like a gas and a liquid. It spreads within a container like a gas, but has a density that is similar to a liquid's. The relatively low process temperature and the stability of carbon dioxide prevent most compounds from being structurally damaged or denatured during extraction. Additionally, the solubility of most compounds obtained with supercritical CO2 extraction varies with pressure, which enables high extraction selectivity.

Extraction with nontoxic materials
Extraction with supercritical fluids is relatively simple and much more efficient than conventional extraction methods, which require heating and as a consequence generate emissions. Supercritical fluids enable a continuous extraction process using conventional, inexpensive, and nontoxic materials. The extracted material is isolated from the supercritical fluid (solvent) by simply relieving the pressure. Supercritical fluids can also be used as solvents for substance application and for fabric dyeing, a process that is more or less the opposite of extraction. Due to their availability and low critical temperatures, the most commonly used supercritical solvents are carbon dioxide and water.

Source: SK Škrlj
CO2 extraction occurs when carbon dioxide is exposed to suitable pressure conditions and temperatures. If those conditions are met, CO2 enters its supercritical state, which means that it has liquid and gas properties.
|
The atmospheric pressure on Venus is about 90 times greater than that on Earth. The average temperature is 467 °C and about 97 % of its atmosphere consists of carbon dioxide. Therefore, the atmosphere of Venus could be considered a supercritical fluid, as both the pressure and temperature exceed the critical point of carbon dioxide, but this theory has never been proven. Similar examples can be found throughout the solar system, especially on the gas giants (Jupiter, Saturn, Uranus, Neptune). |
Source: SK Škrlj
Environmentally friendly solvent for dry cleaning
Supercritical CO2 is used as an extraction solvent for the preparation of essential oils and other herbal distillates. Its main advantages compared to other solvents (e.g. hexane or acetone) are that it is nontoxic and nonflammable. Compared to steam distillation, supercritical CO2 extraction is carried out at lower temperatures and can be used to separate plant waxes from oils. Supercritical CO2 can also be used for dry cleaning as a more environmentally friendly alternative to traditional solvents.
The safest CO2 extraction system
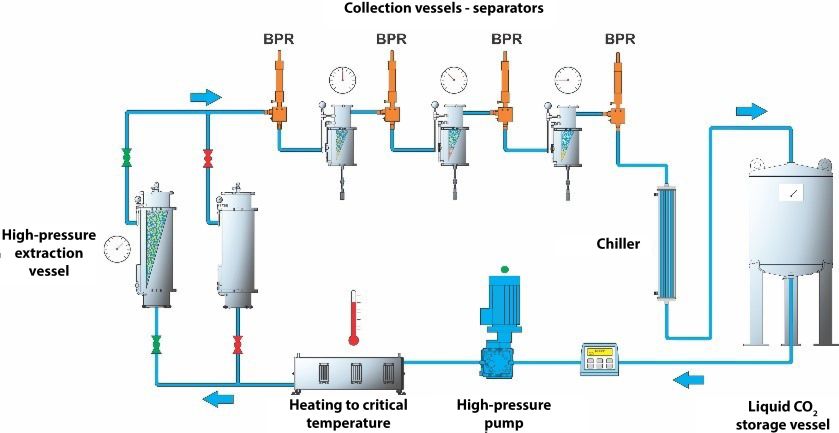
Supercritical CO2 extraction can be carried out using simple manually controlled systems, which require a lot of knowledge and qualified staff. A safer and more efficient alternative are automated extraction systems, which are used by highly qualified experts in various development laboratories and production plants, as they enable process repeatability and at the same time ensure safe working conditions. A supercritical CO2 extraction system consists of a high-pressure pump, extraction vessels and a series of collection vessels. When the CO2 enters its supercritical state, it is pumped through the extraction vessel containing plant material from which the substances will be extracted.

Source: SK Škrlj
The most important advantage of this extraction method is its flexibility. The system pressure and temperature can be adjusted, which enables the extraction of various compounds. Although these systems initially require a high financial investment, they ensure a minimal environmental impact and present a safe and effective alternative to existing environmentally controversial technologies.
First Slovenian supercritical extraction system
Significant advances in the supercritical technology in recent years have stimulated the extraction of extracts from natural materials, including plants and their residues, algae and microalgae. One such system was developed by a team of experts at SK Škrlj, led by scientist and entrepreneur Marko Likon, Ph.D., a leader in the field of eco-innovation. Today, Škrlj d.o.o. is a valued European company with an established international market that specializes in the production of stainless steel process equipment for the winemaking, beer brewing, and pharmaceutical industries.
